 |
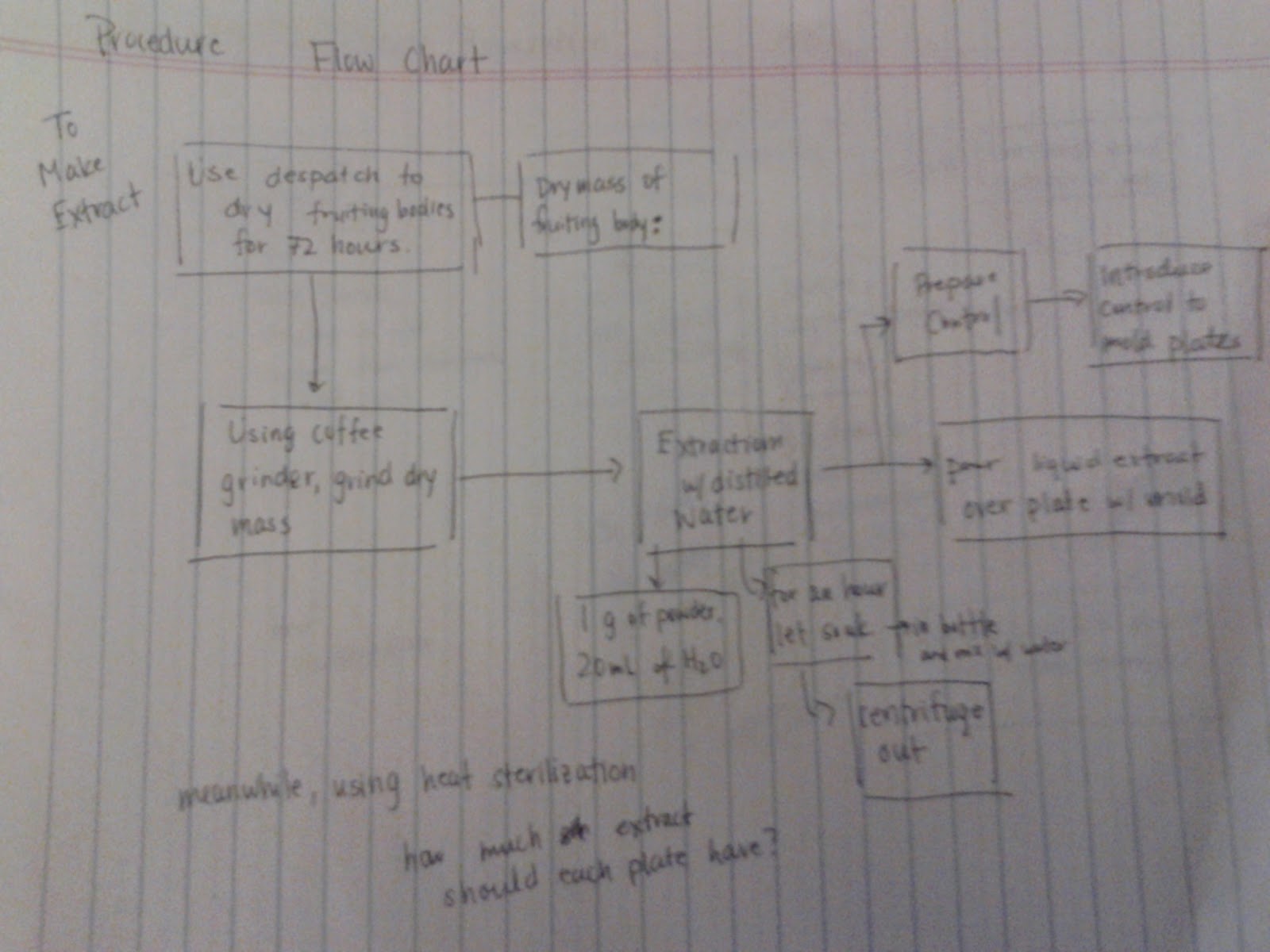
| One of my flow charts |
After sorting corn, I went to work on designing the procedure of my experiment. From my research, I knew the general outline that I had to follow, but the details were vague. I didn't know what equipment was available to me, and since I want to work with a pathogen, how was I going to do this without contaminating the whole lab? Courtney clarified many of these details and suggested that I utilize a flow chart to help organize my thoughts and details. The flow chart especially helps with figuring out the timing. What else could I be doing when the mushrooms are drying or how long does it take to centrifuge? Since I have limited time, this is crucial.
My final experiment will be comparing the antibacterial properties of Pleurotus ostreatus and Coprinus cinereus on Aspergiillus niger.
My first DoE, the one looking to compare light and dark reactions came out with data. Based off of the data for the fruiting bodies wet weight, the light mushrooms had the greatest average wet weight. This is not what we predicted but it is helpful to know nonetheless.





