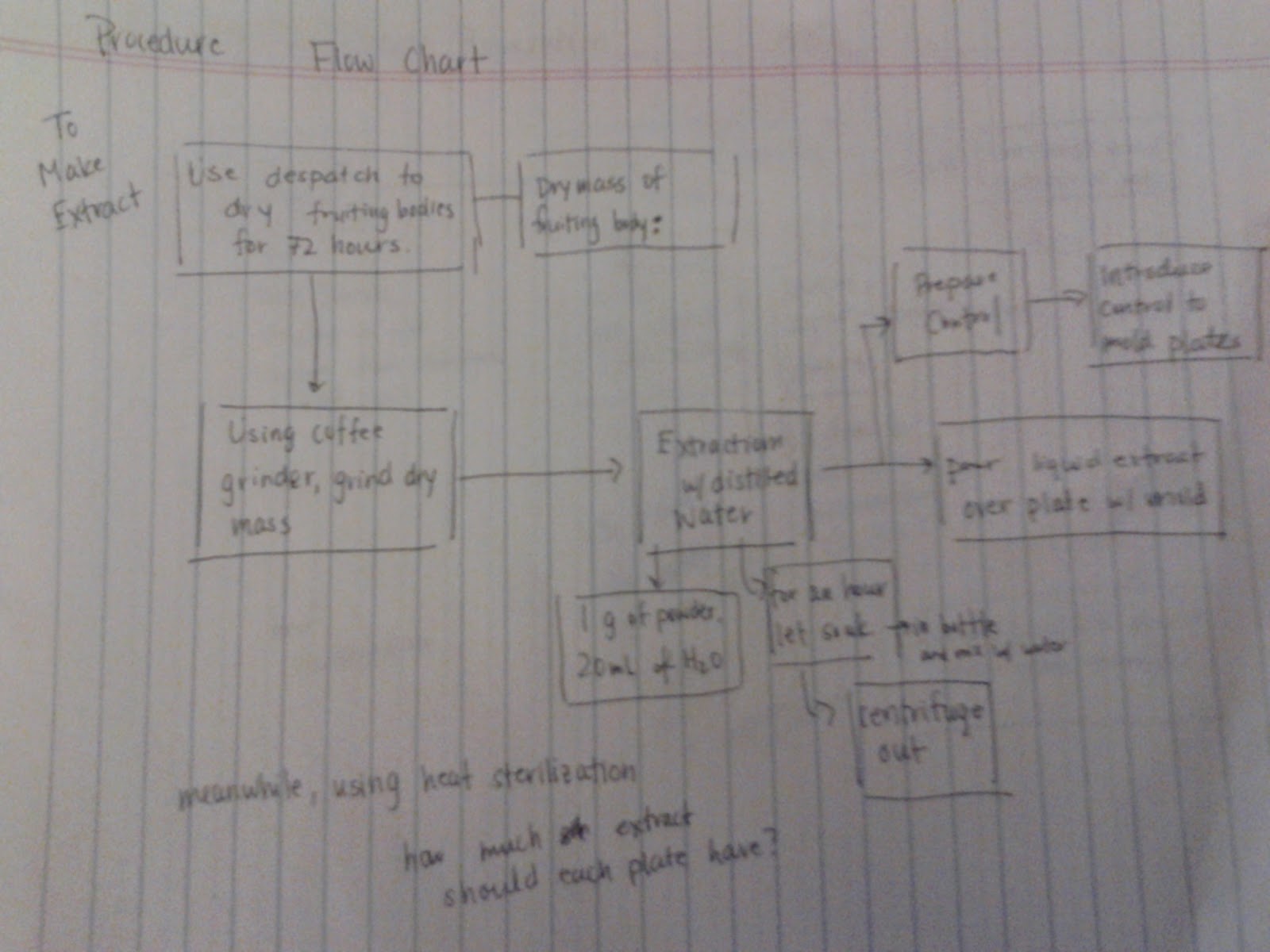
Throughout my whole experience, I really got a strong feel for
what working professionally in a laboratory is like. It is an experience
way different than the labs we do in school. I learned the first steps to
developing a good aseptic technique in the lab. If I want to
work in more research labs, this is an important technique to develop.
This is one of my goals I had hoped to accomplish at the beginning of the
year.
I also found the opportunity to design my own experiments.
While in sciences classes, we have designed experiments as a class, it is
not the same when it comes down to yourself and the word document. I did
have my mentors there to help me, but I was faced with numerous questions that
needed answers before further steps could be taken. Questions such as
what tools were needed and what precise measurements were needed. These
details are just details, but in the end they make a big difference in your
results.
Furthermore, I learned so much about mushrooms and fungi. I
never really understood how complicated this whole kingdom is. In my
mind, they were mostly just a food group. What I knew about mushrooms
could be summed up in two or three sentences. Now, while I don't know as
much about mushrooms as my mentors do, I have a small understanding of how
mushrooms are structurally and how those components can help make improvements
and advancements in science and technology.
Reading scientific papers was challenging experience. In the
beginning, I had to learn how to read the papers and understand them.
Even now, there are a bunch of papers that are harder to read. This
was because there were a lot of terms and concepts I weren't familiar with.
Measurement notation was even strange to read. Ways a tackled this
problem included using Wikipedia and a dictionary. However, the more
papers I read, the more familiar I became with many of the terms.
While learning to read papers was difficult, the end result of
learning new things was the most pleasant. Learning about mycoremediation
to different mushroom species to how a mushroom can regenerate nerve ends was
all rewarding, even if getting through papers was difficult.
Suggestions:
I can't really think of any suggestions. Knowing
presentation/poster dates a little sooner would be nice.
Some advice for next year's interns...
If you are reading a scientific paper last minute, read the
introduction and then the conclusion.
When you begin to first work in a laboratory, its ok to be
paranoid about contaminating everything. Actually, you should be paranoid
about contaminating anything. That way you'll develop good aseptic
techniques.
Never hesitate to ask questions. When you are stuck or
especially when you are curious.